Digital Microfluidics
In this research, I explore the integration of digital microfluidic (DMF) platform into biosensor for point-of-care testing in an automated and sensitive manner. By deploying cell-based/protein-based immunoassays to the electrochemical biosensors
Digital microfluidics (DMF) has gained attention as an ideal candidate for point-of-care (POC) testing due to its liquid manipulation technology that can manipulate droplets on a planar electrode surface under applied voltage based on the electrowetting-on-dielectric (EWOD) principle. DMF devices can operate droplets in an automated and programmable way with minimally trained individuals, and they can be reconfigured on-demand for different applications such as immunological assays, cell-based assays, and DNA amplification. Electrical-based detection is a promising sensing strategy that can be integrated into DMF platforms due to the convenience of integrating electrical sensors into systems composed of electrode arrays. The merging of electrical-based sensors and DMF platforms has enabled various POC applications, particularly immunoassays. So far, electrical sensors have been integrated into DMF platforms for the detection of antigen molecules and rubella virus with high sensitivity and selectivity. These detections are usually based on enzyme-linked immunoassay (ELISA), which immobilizes target molecules on solid surfaces such as magnetic beads and labels captured molecules with enzyme-tagged secondary antibodies. Targets can be quantified by measuring their electrochemical oxidation in the presence of redox probes via amperometric or potentiometric transduction principles using electrochemical electrodes housed on the DMF substrates.
Article 1
Yuqian Zhang. “Advances in integrated digital microfluidic platforms for point-of-care diagnosis: a review.” A review paper describing the current technologies of integrated digital microfluidic platforms.
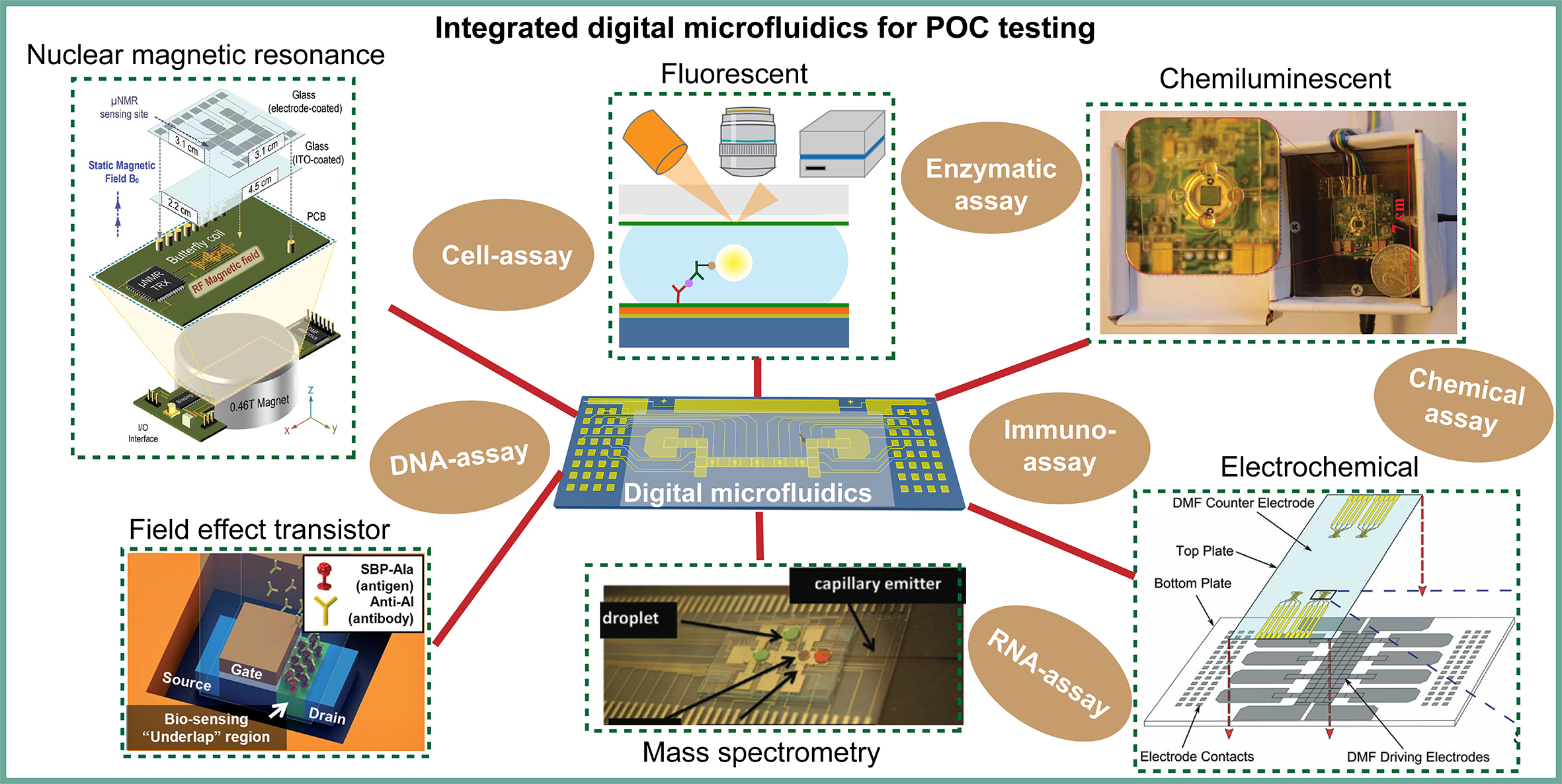
Prompt, reliable and specific detection techniques in portable and easy-to-operate systems are of paramount importance to medical diagnosis, especially in emergencies such as pandemic outbreaks or in resource-limited settings. Point-of-care (POC) testing platforms can offer accurate screening in a timely manner, making these tools ideal under these circumstances. Digital microfluidics (DMF) is a fluid handling technology that enables programmable manipulation of discrete droplets (picoliter to microliter range) on a planar surface featured with electrodes, by changing the surface tension of droplets using electric fields. This technology allows user-defined droplet manipulation such as dispensing, mixing, splitting and merging, and thus the platform can be reconfigured for various assays. Although efforts have been undertaken to optimize the accuracy of fluid handling in DMF devices, implementing these devices for POC testing requires the integration of various detection techniques for on-chip assays. In this review, we highlight recent advancements in the integration of analytical tools into DMF devices, and discuss the current challenges and potential solutions as well as future outlooks for an automated, integrative platform for POC applications.
Integrated digital microfluidic platforms
Article 2
Yuqian Zhang. “A Digital Microfluidic Device Integrated with Electrochemical Impedance Spectroscopy for Cell-Based Immunoassay.” A research reporting an integrated DMF platform for quantifying PBMC abudance.
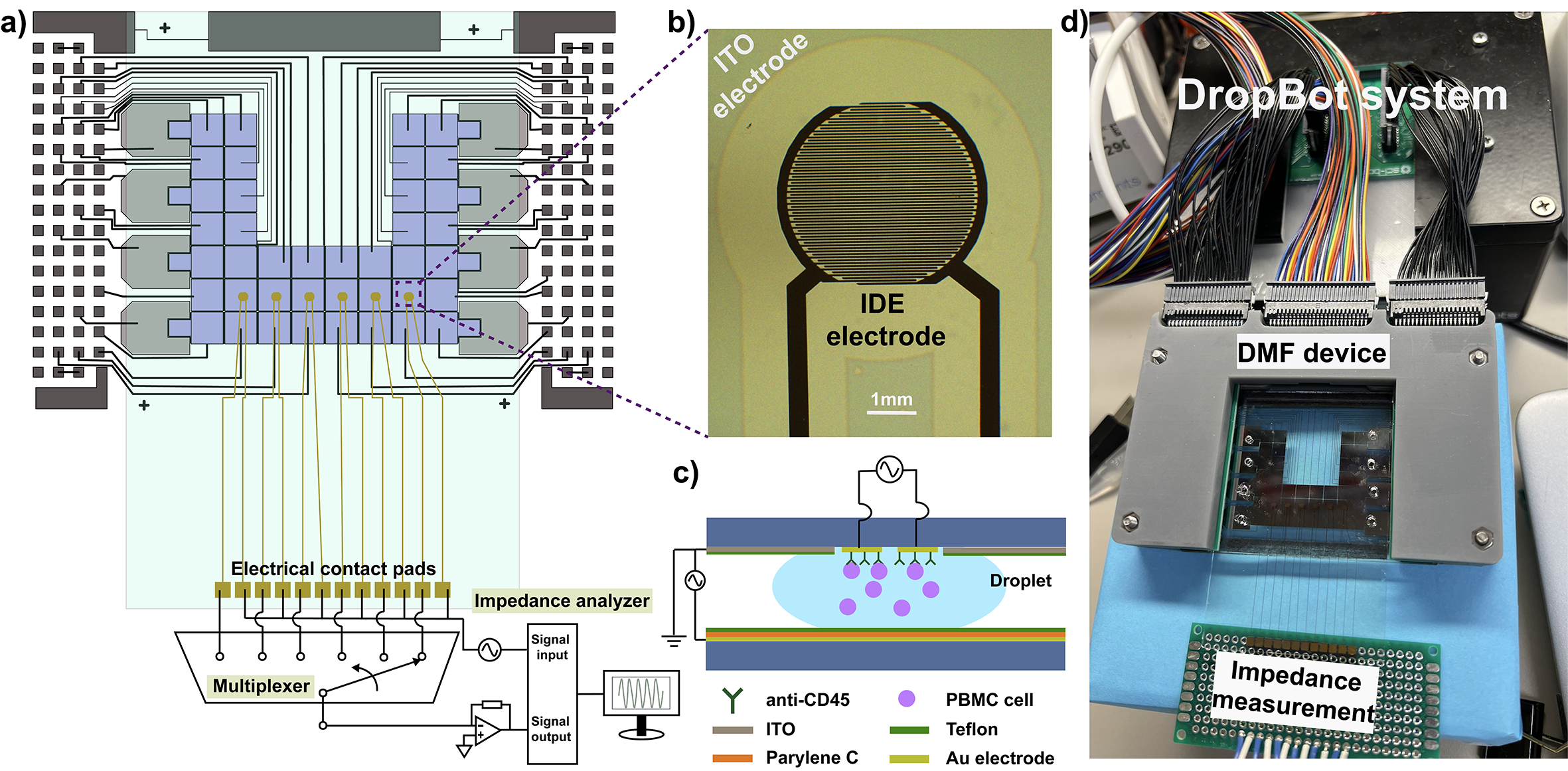
The dynamic immune response to various diseases and therapies has been considered a promising indicator of disease status and therapeutic effectiveness. For instance, the human peripheral blood mononuclear cell (PBMC), as a major player in the immune system, is an important index to indicate a patient’s immune function. Therefore, establishing a simple yet sensitive tool that can frequently assess the immune system during the course of disease and treatment is of great importance. This study introduced an integrated system that includes an electrochemical impedance spectroscope (EIS)-based biosensor in a digital microfluidic (DMF) device, to quantify the PBMC abundance with minimally trained hands. Moreover, we exploited the unique droplet manipulation feature of the DMF platform and conducted a dynamic cell capture assay, which enhanced the detection signal by 2.4-fold. This integrated system was able to detect as few as 104 PBMCs per mL, presenting suitable sensitivity to quantify PBMCs. This integrated system is easy-to-operate and sensitive, and therefore holds great potential as a powerful tool to profile immune-mediated therapeutic responses in a timely manner, which can be further evolved as a point-of-care diagnostic device to conduct near-patient tests from blood samples.
Integrated digital microfluidic platforms
Manuscript in preparation
Yuqian Zhang. “Integrating a 3D matrix structure into a digital microfluidic device for electrochemical detection of soluble PD-L1”
Regularly evaluating therapeutic responsiveness to cancer immunotherapies, such as the level of PD-L1 molecules in patients receiving PD1/PD-L1 immune checkpoint inhibitors, is crucial to maximize the benefit of cure and minimize adverse effects. The gold standard, ELISA, faces logistic challenges in frequent monitoring due to the need for trained technologists in centralized facilities. We integrate an electrochemical sensor array into a digital microfluidic (DMF) device; this platform can be programmed to handle sample droplets automatedly, facilitating the rapid quantification of PD-L1 with minimal training. To improve detection sensitivity, we integrate a porous and electrically conductive 3D matrix structure that contains reduced graphene oxide (rGO), bovine serum albumin (BSA), and glutaraldehyde (GA) onto the sensing electrodes, which increases binding sites and reduces non-specific binding, thus optimizing the immunoassay performance. The mixture of rGO:BSA:GA was drop-cast onto the sensor surface, followed by forming a sandwich immunoassay containing capture antibody, PD-L1 molecules, and a secondary antibody labeled with HRP. The electrochemical signals from the HRP-TMB reaction were then analyzed quantitatively. In this on-chip immunoassay, sample droplets were effectively moved across adjacent actuation electrodes, facilitating dynamic incubation and enhancing the detection sensitivity of PD-L1 to as low as 1 pg/mL.
Yuqian Zhang. “An automated microfluidic platform integrated with electrochemical biosensor to detect circulating PD-L1 extracellular vesicles “
Circulating PD1/PD-L1 checkpoint inhibitors are increasingly acknowledged as non-invasive biomarkers to evaluate the therapeutic responsiveness in tumor Immunotherapy. However, standard measurement assay such as ELISA pose logistical challenges in closely monitoring patients. Therefore, we have developed a digital microfluidic device integrated with electrochemical biosensor to extract tumor derived extracellular vesicles from cell culture media, followed by quantitative detection of the PD-L1 expression level on EVs. This integrated system enables a rapid and automated operation, making it highly suitable to monitor the dynamic changes of PD-L1 markers on a regular basis. We performed an immunomagnetic assay on DMF device in a simple and programmed manner. Droplets containing magnet beads were manipulated to specifically capture EVs from 20 uL of culture media from MDA-MB-231 cell line. The captured EVs were then labeled with PD-L1 detection antibody, followed by HRP conjugation. The assay complex was detected with the electrochemical sensor configured on the top plate of the DMF device1. Result showed this integrated device was able to extract and quantitatively detect the PD-L1 expression levels on EVs with series dilution rates (10-10,000) extracted from cell culture media with good linearity (R² = 0.9176), and the whole procedure can be finished within 2 hours.
