Nanopore-based biosensor
Nanopores present a highly advantageous biosensor platform. A key advantage is their label-free detection capability, eliminating the need for complex and expensive labeling processes. Their high sensitivity enables the detection of individual biomolecules, making them exceptionally effective in various biosensing applications. Additionally, nanopores enable real-time analysis, allowing for the continuous monitoring of dynamic interactions between analytes and their targets. This feature proves especially valuable for studying kinetic events and gaining insights into binding mechanisms.
Article 1
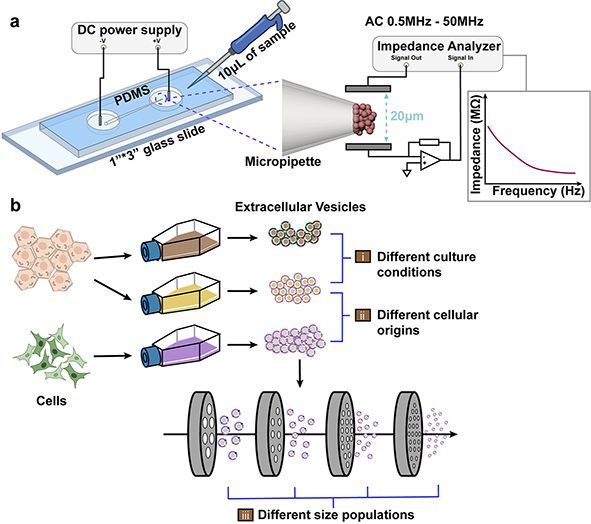
Zhang, Y., Murakami, K., Borra, V.J., Ozen, M.O., Demirci, U., Nakamura, T. and Esfandiari, L.. “A Label-Free Electrical Impedance Spectroscopy for Detection of Clusters of Extracellular Vesicles Based on Their Unique Dielectric Properties” International Studies Quarterly.
Extracellular vesicles (EVs) have gained considerable attention as vital circulating biomarkers since their structure and composition resemble the originating cells. The investigation of EVs’ biochemical and biophysical properties is of great importance to map them to their parental cells and to better understand their functionalities. In this study, a novel frequency-dependent impedance measurement system has been developed to characterize EVs based on their unique dielectric properties. The system is composed of an insulator-based dielectrophoretic (iDEP) device to entrap and immobilize a cluster of vesicles followed by utilizing electrical impedance spectroscopy (EIS) to measure their impedance at a wide frequency spectrum, aiming to analyze both their membrane and cytosolic charge-dependent contents. The EIS was initially utilized to detect nano-size vesicles with different biochemical compositions, including liposomes synthesized with different lipid compositions, as well as EVs and lipoproteins with similar biophysical properties but dissimilar biochemical properties. Moreover, EVs derived from the same parental cells but treated with different culture conditions were characterized to investigate the correlation of impedance changes with biochemical properties and functionality in terms of pro-inflammatory responses. The system also showed the ability to discriminate between EVs derived from different cellular origins as well as among size-sorted EVs harbored from the same cellular origin. This proof-of-concept approach is the first step towards utilizing EIS as a label-free, non-invasive, and rapid sensor for detection and characterization of pathogenic EVs and other nanovesicles in the future.
Article Supplemental Information
Schematic of an integrated iDEP and EIS system
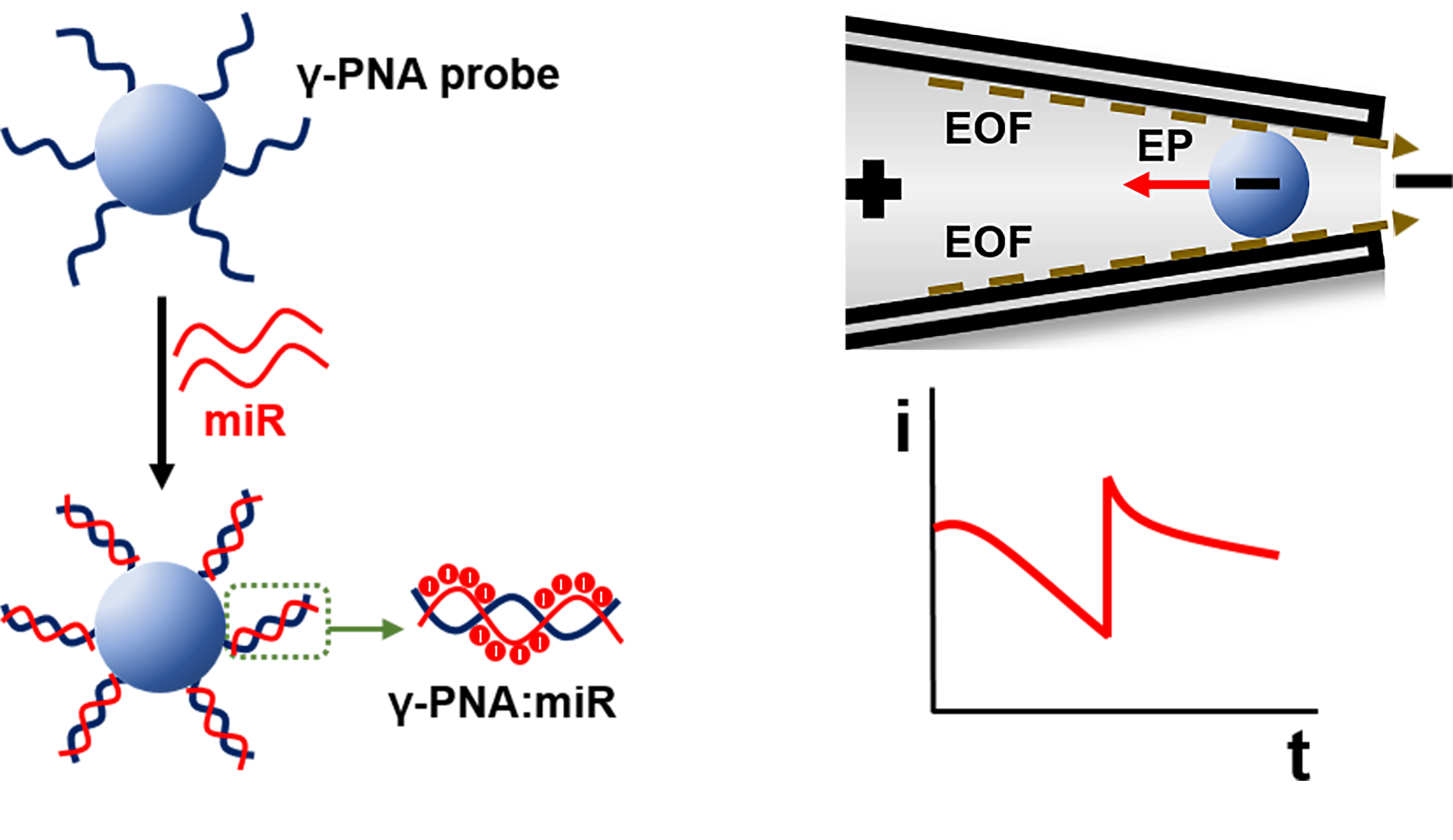
Article 2
Zhang, Y., Rana, A., Stratton, Y., Czyzyk-Krzeska, M.F. and Esfandiari, L.. “Sequence-Specific Detection of MicroRNAs Related to Clear Cell Renal Cell Carcinoma at fM Concentration by an Electroosmotically Driven Nanopore-Based Device” International Studies Quarterly.
MicroRNAs (miRs) are small noncoding RNAs that play a critical role in gene regulation. Recently, traces of cancer-related miRs have been identified in body fluids, which make them remarkable noninvasive biomarkers. In this study, a new nanopore-based detection scheme utilizing a borosilicate micropipette and an assay of complementary γ-peptide nucleic acid (γ-PNA) probes conjugated to polystyrene beads have been reported for the detection of miR-204 and miR-210 related to the clear cell Renal Cell Carcinoma (ccRCC). Electroosmotic flow (EOF) is induced as the driving force to transport PNA-beads harboring target miRs to the tip of the pore (sensing zone), which results in pore blockades with unique and easily distinguishable serrated shape electrical signals. The concentration detection limit is investigated to be 1 and 10 fM for miR-204 and miR-210, respectively. The EOF transport mechanism enables highly sensitive detection of molecules with low surface charge density with 97.6% detection accuracy compared to the conventional electrophoretically driven methods. Furthermore, resistive-pulse experiments are conducted to study the correlation of the particles’ surface charge density with their translocation time and verify the detection principle.
Article Supplemental Information
Sequence-specific detection of small microRNAs
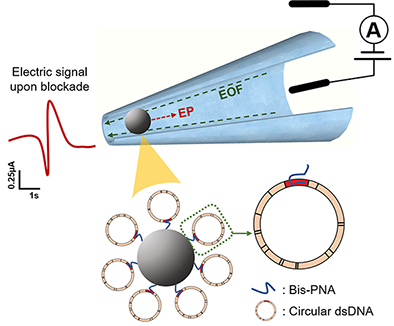
Article 3
Zhang, Y., Kaynak, A., Huang, T. and Esfandiari, L.. “A rapid bioanalytical tool for detection of sequence-specific circular DNA and mitochondrial DNA point mutations” International Studies Quarterly.
Mutations in mitochondrial DNA (mtDNA) have been an essential cause of numerous diseases, making their identification critically important. The majority of mtDNA screening techniques require polymerase chain reaction (PCR) amplification, enzymatic digestion, and denaturation procedures, which are laborious and costly. Herein, we developed a sensitive PCR-free electrokinetic-based sensor combined with a customized bis-peptide nucleic acid (bis-PNA) and gamma-PNA (γ-PNA) probes immobilized on beads, for the detection of mtDNA point mutations and sequence-specific supercoiled plasmid DNA at the picomolar range. The probes are capable of invading the double-stranded circular DNA and forming a stable triplex structure. Thus, this method can significantly reduce the sample preparation and omit the PCR amplification steps prior to sensing. Further, this bioanalytical tool can open up a new paradigm in clinical settings for the screening of double-stranded circular nucleic acids with a single-base mismatch specificity in a rapid and sensitive manner.
Sequence-specific detection of circular DNA